Important Notice

A.D.:
Nepal Samvat: 1146 THIMLAGA CHATURTHI - 19



A.D.:
Nepal Samvat: 1146 THIMLAGA CHATURTHI - 19
June 15, 2025, 12:22 PM
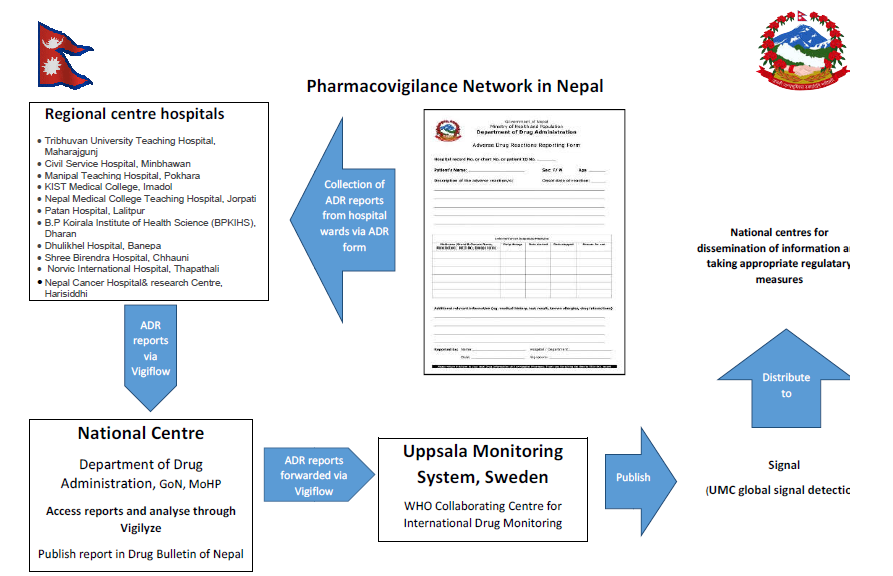
The Government of Nepal nominated Department of Drug Administration (DDA) in October 2004 as the focal point (National Pharmacovigilance centre) to liaison with WHO collaborating centre for International Drug Monitoring, Sweden and started collecting adverse drug reactions. Nepal became a WHO programme member in July 2006. In continuation to 15 regional pharmacovigilance centers, the department recently recognized 4 additional hospitals in Makwanpur, Chitwan, Bhaktapur, and Kathmandu to further strengthen the pharmacovigilance program.
At present, there are 19 regional pharmacovigilance centers in Nepal
These regional pharmacovigilance centers operate under DDA (DDA being the National centre for ADR monitoring). The regional centers reports ADRs to the National center (DDA) via ‘Vigiflow’ (an online program) which are then forwarded to the Uppsala Monitoring Center (UMC) by the National Centre. The National database contains about 1204 ADR reports so far.